Supporting Breakthrough Treatments
Supporting Breakthrough Treatments
Today, we know more about the causes of diseases than ever before. Yet treatments and cures are lacking for more than 500 million people worldwide.
According to the National Institutes of Health (NIH), 80 to 90% of research projects fail before reaching testing in humans. Pharmaceutical companies and venture capital investors are understandably discerning, favoring projects with proven results and replicability.
The gap between promising scientific discoveries and new medicines has been widening for at least 20 years, during which funding for translational research – research that verifies therapeutic targets – has been dissipating.
Strategic philanthropy can fill the gap by supporting efforts to achieve early-stage results where many projects tend to languish and lose attention because of investment risk.
CHARTING THE PATH FORWARD
Great medical breakthroughs exist in academic medical centers around the world. However, they often require additional resources and expertise to advance out of the lab. Let’s review the drug development phases and steps shown below.
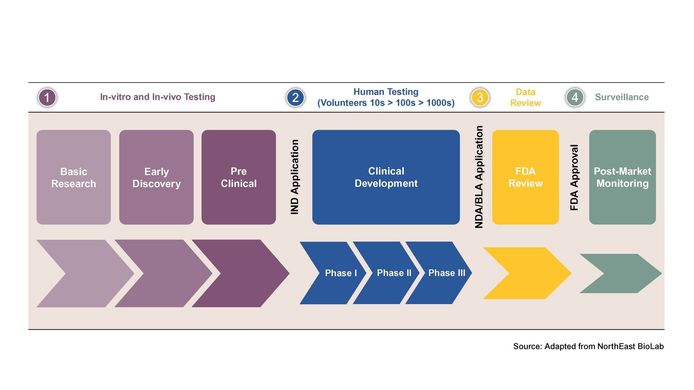
Basic research and early discovery
This phase encompasses studying the underlying cause(s) of a disease, identifying a therapeutic target, publishing the findings along with a hypothesis for treatment. Data may be obtained from in-vitro (cells in a petri dish) or in-vivo testing (animals with the human disease).
Once a target has been identified, finding a promising therapeutic involves a costly process of screening perhaps thousands of compounds – natural products, synthetic chemicals, or biologics (such as antibodies or peptides). The compounds that show promising activity are called drug leads. Philanthropic funding can support medicinal chemistry “design,” as well as rapid screening costs.
This step typically takes several years.
Pre-clinical
Drug compounds are evaluated in living organisms. The investigations are conducted by researchers or a contract research organization (CRO). These studies generate detailed efficacy and metabolic data, as well as safety data including any toxicity issues that could prevent the drug from progressing to a drug candidate and clinical (human) trials.
Clinical development
If a drug candidate shows promising efficacy and safety results, an investigational new drug (IND) application is submitted to the regulatory agency (Food and Drug Administration (FDA) in the U.S.). Once approved, human studies, typically using volunteers, are designed and conducted in three phases:
- Phase I – safety testing, typically with a small number of healthy volunteers
- Phase II – efficacy and optimal dosing in a large group of patients
- Phase III – confirming the drug’s efficacy and safety in an expanded group of patients in a real-world setting
Clinical development can take several years and only a few drug candidates will pass all three phases.
Regulatory review of data
Once clinical trials are complete, it’s time to submit a new drug application (NDA) to a regulatory agency, such as the FDA, for approval. The regulatory agency conducts its own review to evaluate benefits and risks of the drug for the intended population and decides whether to approve or reject it. This step can take several months or years depending on the complexity of the data and the availability of expedited pathways.
If the drug receives FDA approval, it can be commercialized, meaning it can be marketed, distributed, and prescribed to patients.
Post-market monitoring
The FDA monitors all drug and device safety, reviews reports of problems with prescription and over-the-counter drugs, and decides whether to add cautions to the dosage or usage information, as well as other measures for more serious issues.
THE POWER OF PHILANTHROPY
Philanthropy is playing a unique and critical role in supporting translational medicine aimed at advancing more candidates to drug development. Funding from nonprofits can support CRO studies, academic research, project management and commercialization, sustaining work beyond hurdles, which are otherwise difficult to surmount. For instance, flexible funding has emerged as a strategy to support early-stage research where many projects stumble. It removes preliminary reporting burdens researchers face in making medicine, allowing them to focus on science.
Importantly, philanthropic institutions can encourage knowledge exchange and resource sharing between “siloed” academic, pharma industry, and healthcare institutions. The result of such collaboration builds the bridge to translate scientific breakthroughs into medical treatments that improve and save lives.
HARRINGTON IMPACT
Harrington Discovery Institute provides scientists with funding and an advisory team with experience bringing new drugs to market. The Harrington team brings expertise to each phase of drug development. In just over ten years, Harrington has supported the advancement of 177 scientific breakthroughs across the US, Canada, and the UK.
Examples of Harrington-supported medicines-in-the-making and their drug development stage:
- Early Discovery Creating a special molecule to help fight Alzheimer's disease. (Xin Qi, PhD, Case Western Reserve University, 2022 Vinney Scholar)
- Pre-clinical A universal anti-flu drug. (Jeffrey Glenn, MD, PhD, Stanford University, Harrington Scholar-Innovator)
- Pre-clinical A treatment to grow new bones and repair damaged bones to prevent and treat osteoporosis. (Marc Wein, MD, PhD, Massachusetts General Hospital, Harrington Scholar-Innovator)
- Clinical Development Phase III A topical treatment to prevent and treat skin cancer. (Jean Tang, MD, PhD, Stanford University, Harrington Scholar-Innovator)